Abstract
Introduction: Prognosis of AL amyloidosis has improved in recent years; however for many patients prognosis remains poor. We aimed to define patient-, disease- and treatment characteristics which are associated with long-term survival.
Method: A retrospective chart review of all patients with biopsy-proven systemic AL amyloidosis, who were seen within 90 days of the confirmed diagnosis. Long-term survival was defined as 5-year and 10-year survival from the time of diagnosis. For 5-year survival we selected patients seen between January 1, 2000 and December 31, 2012 (allowing a minimum of 5-year follow-up, n=1331) and for 10-year survival we screened patients seen between January 1, 2000 and December 31, 2007 (allowing a minimum of 10-year follow-up; n=779). Treatment allocation was defined as the first regimen given, irrespective of subsequent treatment modifications.
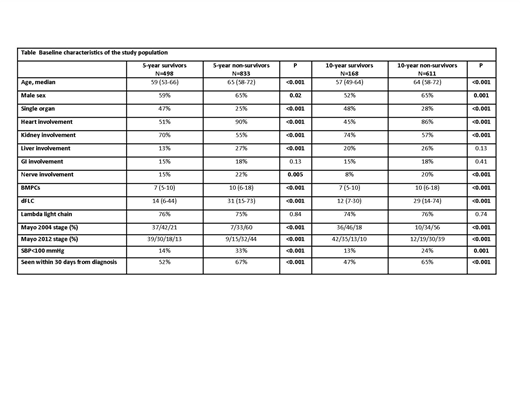
Results: Of the screening population, 498 patients survived ≥5 years from diagnosis (37% of the 5-year screening cohort) and 168 patients survived 10 years or more (22% of the 10-year screening cohort). Five-year survivors and 10-year survivors as compared to their counterparts were (Table): younger, higher proportion of women, more likely to have single organ involvement, less heart/liver/nerve involvement and more kidney involvement. Long-term survivors also had lower bone marrow plasma cell percentage at the time of diagnosis and lower tumor burden measured by the difference between involved to uninvolved light chain (dFLC). Similarly, long-term survivors had lower Mayo stages and higher systolic blood pressure. No difference in light chain isotype was observed between long-term survivors to long term non-survivors. Long-term survivors were less likely to be seen within 30 days of diagnosis compared to their counterparts (52% among 5-year survivors vs 67% among 5-year non-survivor; P<0.001). FISH abnormalities (data available for 555/1331 patients, 42%) were comparable between groups with regard to t(11;14) (50% among 5-year survival compared to 50% among 5-year non-survivors; P=0.93) and 13q abnormalities (34% vs 36%, respectively; P=0.53). However, trisomies were less frequently encountered in the 5-year survivor group (20% vs 29%, respectively; P=0.01), and far less common among 10-year survivors (11% vs 26%, respectively; P=0.04).
Autologous stem cell transplantation (ASCT) was more likely to be associated with long-term survival. Of all patients who underwent ASCT, 74% survived more than 5 years and 49% survived more than 10 years. In comparison, among the standard-intensity therapies, 5-year survival rates for melphalan-dexamethasone, bortezomib-based regimens, immunomodulatory drug-based regimens and single agent dexamethasone/ melphalan-prednisone were 29%, 28%, 30% and 10%, respectively. The corresponding 10-year survival rates were 15%, 20%, 20% and 5%, respectively.
Conclusions: Long-term AL survivors have distinct favorable baseline characteristics (including those introduced by referral bias) and ASCT as their initial therapy. Identification of these patients, especially the Mayo 2004 stage III and the Mayo 2012 stage III-IV patients who unexpectedly survived 10 years, will allow for further study and insights.
Gertz:Teva: Consultancy; Prothena: Honoraria; Alnylam: Honoraria; celgene: Consultancy; Ionis: Honoraria; Physicians Education Resource: Consultancy; Research to Practice: Consultancy; Amgen: Consultancy; janssen: Consultancy; Apellis: Consultancy; Medscape: Consultancy; Abbvie: Consultancy; spectrum: Consultancy, Honoraria; annexon: Consultancy. Lacy:Celgene: Research Funding. Dingli:Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Millennium Takeda: Research Funding; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Russell:Vyriad: Equity Ownership. Kumar:KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal